The Chlamydia Infection Diagnostics Global Market Report 2024 by The Business Research Company provides market overview across 60+ geographies in the seven regions – Asia-Pacific, Western Europe, Eastern Europe, North America, South America, the Middle East, and Africa, encompassing 27 major global industries. The report presents a comprehensive analysis over a ten-year historic period (2010-2021) and extends its insights into a ten-year forecast period (2023-2033).
Learn More On The Chlamydia Infection Diagnostics Market:
https://www.thebusinessresearchcompany.com/report/chlamydia-infection-diagnostics-global-market-report
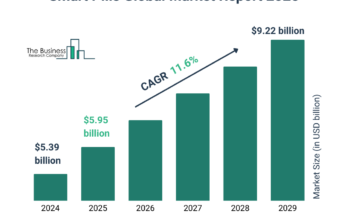
According to The Business Research Company’s Chlamydia Infection Diagnostics Global Market Report 2024, The chlamydia infection diagnostics market size has grown rapidly in recent years. It will grow from $1.12 billion in 2023 to $1.25 billion in 2024 at a compound annual growth rate (CAGR) of 11.3%. The growth in the historic period can be attributed to increasing awareness and education, government funding, increasing prevalence of sexually transmitted infections (STIs), and healthcare accessibility.
The chlamydia infection diagnostics market size is expected to see rapid growth in the next few years. It will grow to $1.94 billion in 2028 at a compound annual growth rate (CAGR) of 11.6%. The growth in the forecast period can be attributed to the growing demand for rapid testing, rising global agency support, government initiatives, growing demand for quick and reliable testing, and increasing prevalence of chlamydia infections. Major trends in the forecast period include advancements in molecular diagnostics, expansion of point-of-care testing (POCT), integration of digital health technologies, focus on population screening and public health initiatives, emerging technologies, and innovations.
The growing focus on preventive healthcare is expected to propel the chlamydia infection diagnostics market going forward. Preventive healthcare involves measures taken to prevent diseases, rather than treating them after they occur, through regular check-ups, vaccinations, and lifestyle modifications. The growing focus on preventive healthcare aims to reduce healthcare costs, improve quality of life, and prevent the onset of chronic diseases through early detection and proactive health measures. Preventive healthcare plays a crucial role in chlamydia diagnostics by emphasizing early detection, education, and routine screening, which collectively help in reducing the spread and complications of chlamydia infections. For instance, in May 2023, according to the National Health Service (NHS), a UK-based publicly funded healthcare system, around 23.02 million diagnostic tests were conducted in March 2023, reflecting an increase of 223,100 tests compared to March 2022. Further, in March 2023, the number of patients waiting for a critical diagnostic test reached 16.28 million, marking an increase of 59,400 compared to March 2022. Therefore, the growing focus on preventive healthcare drives the growth of the chlamydia infection diagnostics market.
Get A Free Sample Of The Report (Includes Graphs And Tables):
https://www.thebusinessresearchcompany.com/sample.aspx?id=18296&type=smp
The chlamydia infection diagnostics market covered in this report is segmented –
1) By Test Type: Culture Test, Nucleic Acid Amplification Test (NAAT), Direct Fluorescent Antibody Test, Serology Test, Other Test Types
2) By Type Of Infections: Genital Chlamydia Infection, Rectal Chlamydia Infection, Ocular Chlamydia Infection
3) By Application: Hospitals, Specialty Clinics, Diagnostic Centers
4) By End User: Diagnostics, Therapeutics
Major companies operating in the chlamydia infection diagnosis market focus on developing advanced products, such as molecular point-of-care testing platforms, to gain a competitive edge. Molecular point-of-care (POC) testing platforms are diagnostic tools designed to detect and analyze specific nucleic acids (DNA or RNA) of pathogens directly at the patient care site. For instance, in March 2021, Binx Health Inc., a US-based health technology company, announced that the US Food and Drug Administration (FDA) had awarded a clinical laboratory improvement amendments (CLIA) waiver for their Binx IO system. The system is a molecular point-of-care testing platform that can provide central laboratory-quality results in approximately 30 minutes for detecting chlamydia (CT) and gonorrhea (NG). The platform had already received 510(k) clearance from the FDA for testing male and female samples in moderate and high-complexity settings.
The chlamydia infection diagnostics market report table of contents includes:
1. Executive Summary
2. Chlamydia Infection Diagnostics Market Characteristics
3. Chlamydia Infection Diagnostics Market Trends And Strategies
4. Chlamydia Infection Diagnostics Market – Macro Economic Scenario
5. Global Chlamydia Infection Diagnostics Market Size and Growth
.
.
.
32. Global Chlamydia Infection Diagnostics Market Competitive Benchmarking
33. Global Chlamydia Infection Diagnostics Market Competitive Dashboard
34. Key Mergers And Acquisitions In The Chlamydia Infection Diagnostics Market
35. Chlamydia Infection Diagnostics Market Future Outlook and Potential Analysis
36. Appendix
Contact Us:
The Business Research Company
Europe: +44 207 1930 708
Asia: +91 88972 63534
Americas: +1 315 623 0293
Email: info@tbrc.info
Follow Us On:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
Twitter: https://twitter.com/tbrc_info
Facebook: https://www.facebook.com/TheBusinessResearchCompany
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Blog: https://blog.tbrc.info/
Healthcare Blog: https://healthcareresearchreports.com/
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model