The acquired hemophilia treatment global market report 2024 from The Business Research Company provides comprehensive market statistics, including global market size, regional shares, competitor market share, detailed segments, trends, and opportunities. This report offers an in-depth analysis of current and future industry scenarios, delivering a complete perspective for thriving in the industrial automation software market.
Acquired Hemophilia Treatment Market, 2024 report by The Business Research Company offers comprehensive insights into the current state of the market and highlights future growth opportunities.
Market Size –
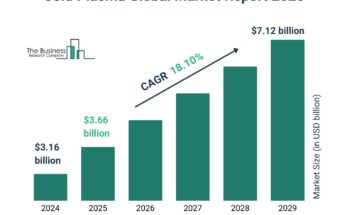
The acquired hemophilia treatment market size has grown strongly in recent years. It will grow from $11.47 billion in 2023 to $12.2 billion in 2024 at a compound annual growth rate (CAGR) of 6.3%. The growth in the historic period can be attributed to increasing ageing population, improved diagnostics and awareness, expanding patient pool, collaborative research initiatives, regulatory support and approvals..
The acquired hemophilia treatment market size is expected to see strong growth in the next few years. It will grow to $14.88 billion in 2028 at a compound annual growth rate (CAGR) of 5.1%. The growth in the forecast period can be attributed to growing global population, rising healthcare investments, personalized medicine trends, international collaborations in research, increasing healthcare awareness, regulatory support for orphan drugs.. Major trends in the forecast period include enhanced monitoring and diagnostic technologies, collaboration for patient support programs, research on gene therapy approaches, increased utilization of immune tolerance induction, telemedicine for remote consultations..
Order your report now for swift delivery @
https://www.thebusinessresearchcompany.com/report/acquired-hemophilia-treatment-global-market-report
Scope Of Acquired Hemophilia Treatment Market
The Business Research Company’s reports encompass a wide range of information, including:
1. Market Size (Historic and Forecast): Analysis of the market’s historical performance and projections for future growth.
2. Drivers: Examination of the key factors propelling market growth.
3. Trends: Identification of emerging trends and patterns shaping the market landscape.
4. Key Segments: Breakdown of the market into its primary segments and their respective performance.
5. Focus Regions and Geographies: Insight into the most critical regions and geographical areas influencing the market.
6. Macro Economic Factors: Assessment of broader economic elements impacting the market.
Acquired Hemophilia Treatment Market Overview
Market Drivers –
A growing number of ongoing clinical trials is expected to propel the growth of the acquired hemophilia treatment market going forward. Clinical trials are research studies conducted with human participants to evaluate the safety and effectiveness of new medical treatments, interventions, or diagnostic procedures. Clinical trials assess the safety and efficacy of new treatments for acquired hemophilia, advancing research to improve patient outcomes and establish effective therapeutic interventions. For instance, in October 2023, according to ClinicalTrials.gov, a part of the National Institutes of Health (NIH), a US-based government agency responsible for conducting and supporting medical research, the number of registered studies increased from 437,523 in 2022 to 468,457 in all 50 states of the United States and across 221 countries. Moreover, it was reported that 143,613 studies (31% of the total) are registered in the U.S. only, while 251,159 studies (54% of the total) are registered in non-U.S. locations. Therefore, a growing number of ongoing clinical trials is driving the growth of the acquired hemophilia treatment market.
Market Trends –
Major companies operating in the acquired hemophilia treatment market are focused on providing drugs with additional measures and getting them approved to sustain their position in the market. Drug approvals to include routine prophylaxis are increasing, mainly due to the need for more effective and safer. For instance, in June 2022, Chugai Pharmaceutical, a Japan-based drug manufacturer received extended approval from Japan’s Ministry of Health, Labor and Welfare, to expand its use of Hemlibra (emicizumab) to include routine prophylaxis measures aimed at reducing or minimizing the occurrence of bleeds in individuals with acquired hemophilia A. Following this approval, additional measures to promptly manage and control bleeding shortly after diagnosis were allowed using Hemlibra, a bispecific antibody that binds simultaneously to two key targets, factor IX and factor X binding sites in the blood clotting process.
The acquired hemophilia treatment market covered in this report is segmented –
1) By Treatment: On-Demand, Prophylaxis
2) By Type: Hemophilia A, Hemophilia B, Hemophilia C, Other Types
3) By End User: Hospitals, Clinic, Other End Users
Get an inside scoop of the acquired hemophilia treatment market, Request now for Sample Report @
https://www.thebusinessresearchcompany.com/sample.aspx?id=13363&type=smp
Regional Insights –
North America was the largest region in the acquired hemophilia treatment market in 2023. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the acquired hemophilia treatment market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
Key Companies –
Major companies operating in the acquired hemophilia treatment market report are Pfizer Inc., Roche Holding AG , Bayer AG, Novartis AG, Bristol-Myers Squibb Company, Sanofi SA, GlaxoSmithKline plc, Takeda Pharmaceutical Company Limited, Amgen Inc., Novo Nordisk A/S, Baxter Healthcare Corporation, Teva Pharmaceutical Industries Ltd., Mylan NV, Biogen Inc., CSL Limited , Grifols SA, Genentech Inc., Octapharma AG, Ferring Pharmaceuticals BV, Shire plc, BioMarin Pharmaceutical Inc., Sobi Inc., Kedrion Biopharma Inc., Chugai Pharmaceutical Co. Ltd., Spark Therapeutics Inc., Medexus Pharmaceuticals Inc., UniQure NV, Catalyst Biosciences Inc., Hema Biologics LLC, BioXcel Corporation
Table of Contents
1. Executive Summary
2. Acquired Hemophilia Treatment Market Report Structure
3. Acquired Hemophilia Treatment Market Trends And Strategies
4. Acquired Hemophilia Treatment Market – Macro Economic Scenario
5. Acquired Hemophilia Treatment Market Size And Growth
…..
27. Acquired Hemophilia Treatment Market Competitor Landscape And Company Profiles
28. Key Mergers And Acquisitions
29. Future Outlook and Potential Analysis
30. Appendix
Contact Us:
The Business Research Company
Europe: +44 207 1930 708
Asia: +91 88972 63534
Americas: +1 315 623 0293
Email: [email protected]
Follow Us On:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
Twitter: https://twitter.com/tbrc_info
Facebook: https://www.facebook.com/TheBusinessResearchCompany
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Blog: https://blog.tbrc.info/
Healthcare Blog: https://healthcareresearchreports.com/
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model