atrial septal defect market growth, atrial septal defect market outlook, atrial septal defect market top players, atrial septal defect market segments, atrial septal defect market demand, atrial septal defect market trends
What are the recent trends in market size and growth for the atrial septal defect market?
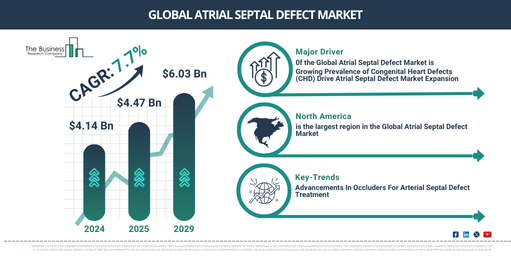
The atrial septal defect market size has grown strongly in recent years. It will grow from $4.14 billion in 2024 to $4.47 billion in 2025 at a compound annual growth rate (CAGR) of 8.0%. The growth in the historic period can be attributed to rise in number of congenital heart disease cases, increased funding for research and development of ASD treatments, high awareness about congenital heart defects among parents and healthcare providers, higher rates of pediatric surgeries, approval of new ASD closure devices and therapies by regulatory bodies like the FDA, expansion of healthcare facilities.
The atrial septal defect market size is expected to see strong growth in the next few years. It will grow to $6.03 billion in 2029 at a compound annual growth rate (CAGR) of 7.7%. The growth in the forecast period can be attributed to increased use of telemedicine for pre and post-operative care, growth in personalized treatment plans based on genetic and phenotypic data, continued increase in global healthcare spending, particularly in emerging markets, growing elderly population leading to a higher incidence of congenital heart defects being diagnosed later in life international harmonization of medical device regulations improving global market penetration, implementation of national and international health programs targeting early detection and treatment of congenital heart diseases. Major trends in the forecast period include development of new, minimally invasive ASD closure devices, the use of AI and machine learning for improved diagnosis and personalized treatment planning, introduction of biodegradable and bioresorbable materials for device manufacturing, AI-driven tools for real-time monitoring and decision support during ASD procedures, adoption of remote monitoring technologies to track patient recovery and detect complications early.
Get Your Free Sample of The Global Atrial Septal Defect Market Report:
https://www.thebusinessresearchcompany.com/sample.aspx?id=16345&type=smp
How have varous drivers impacted the growth of the atrial septal defect market?
The prevalence of congenital heart defects (CHD) is expected to propel the growth of the atrial septal defect market going forward. Congenital heart defects refer to structural abnormalities in the heart present at birth. The increasing prevalence of congenital heart defects refers to the growing number of individuals diagnosed with structural heart abnormalities present from birth. Atrial septal defect treatment helps manage congenital heart defects by correcting abnormal blood flow between the heart’s chambers, improving overall heart function, and reducing associated complications. For instance, in June 2024, according to the Australian Institute of Health and Welfare, an Australia-based national agency, in Australia, approximately 65,000 children and adults are living with congenital heart disease. In the 2020–21 period, there were about 5,900 hospitalizations where congenital heart disease was the primary diagnosis, accounting for 79 deaths in infants under one year old, representing 7.8% of all infant deaths. Therefore, the prevalence of congenital heart defects will drive the atrial septal defect market.
What are the primary segments of the atrial septal defect market?
The atrial septal defect market covered in this report is segmented –
1) By Treatment Procedure: Surgical Closure, Transcatheter Closure, Hybrid Procedures, Medication Therapy
2) By Diagnosis: Chest X-Ray, Electrocardiogram, Cardiac Catheterization, Transesophageal Echocardiography, Pulse Oximetry
3) By Product Type: Medical Devices, Pharmaceutical Products
4) By Age Group: Pediatric, Adult
5) By End-User: Hospitals, Ambulatory Surgical Centers, Cardiac Clinics, Other End-Users
Subsegments:
1) By Surgical Closure: Open Heart Surgery (Traditional Surgical Closure), Minimally Invasive Surgical Closure, Patches (Pericardial, Synthetic) For Asd Closure
2) By Transcatheter Closure: Device-Based Closure (Amplatzer Septal Occluder), Transcatheter Plug Closure Techniques, Catheter-Based Asd Repair For Adult And Pediatric Patients
3) By Hybrid Procedures: Combination Of Surgical And Transcatheter Techniques, Minimally Invasive Hybrid Procedures, Hybrid Closure For Complex Or Large Asds
4) By Medication Therapy: Anticoagulant Therapy (To Prevent Stroke), Diuretics (For Managing Symptoms), Antihypertensive Drugs (For Managing Associated Conditions), Off-Label Use Of Other Medications
Order your report now for swift delivery
https://www.thebusinessresearchcompany.com/report/atrial-septal-defect-global-market-report
Which firms are leading the atrial septal defect market?
Major companies operating in the atrial septal defect market are Abbott Laboratories, Medtronic plc, Siemens Healthineers, Boston Scientific Corporation, Philips Healthcare, Edwards Lifesciences Corporation, W. L. Gore & Associates Inc., St. Jude Medical LLC, Cook Medical, Lepu Medical Technology Co. Ltd., MicroPort Scientific Corporation, Lifetech Scientific Corporation, Venus Medtech Hangzhou Inc, Occlutech Holding AG, Coherex Medical, Osypka AG, Arjo AB, Heart Medical Europe BV, AtriCure Inc., Asklepion Pharmaceuticals LLC
How will industry trends affect the trajectory of the atrial septal defect market?
Major companies operating in the arterial septal defect market are developing advanced devices and seeking approval to increase their availability and uses. Device approval refers to obtaining official authorization or certification for a device, typically from a regulatory body or relevant authority, before it can be legally manufactured, sold, or used in a specific market or for a particular purpose. For instance, in March 2024, Occlutech GmbH, a Sweden-based specialist provider of minimally invasive cardiac devices, announced that the United States Food and Drug Administration (FDA) had approved the Occlutech ASD Occluder and Occlutech Pistol Pusher for treating atrial septal defects (ASD). This approval marks a significant milestone for the company, which has been working to advance healthcare globally. The Occlutech ASD Occluder is designed to be a lifelong solution for patients with echocardiography-confirmed defects. It is a self-expanding nitinol device that includes two flexible discs attached to both sides of the patient’s atrial septum using the Occlutech Pistol Pusher. With this approval, Occlutech will begin commercialization in the US through an exclusive partnership with B. Braun Interventional Systems.
Which geographic trends are shaping the atrial septal defect market, and which region has the highest market share?
North America was the largest region in the atrial septal defect market in 2023. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the atrial septal defect market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
What Does The Atrial Septal Defect Market Report 2025 Offer?
The atrial septal defect market research report from The Business Research Company offers global market size, growth rate, regional shares, competitor analysis, detailed segments, trends, and opportunities.
An atrial septal defect (ASD) refers to a congenital heart defect characterized by an abnormal opening in the septum, the wall separating the heart’s two upper chambers (atria). This opening allows oxygen-rich blood from the left atrium to mix with oxygen-poor blood in the right atrium, which can lead to increased blood flow to the lungs and over time cause complications like pulmonary hypertension and heart failure.
Purchase the exclusive report now to unlock valuable market insights:
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=16345
About The Business Research Company
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead.
Our flagship product, the Global Market Model, is a premier market intelligence platform delivering comprehensive and updated forecasts to support informed decision-making.
Contact Us:
The Business Research Company
Europe: +44 207 1930 708
Asia: +91 88972 63534
Americas: +1 315 623 0293
Email: info@tbrc.info
Follow Us On:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
Twitter: https://twitter.com/tbrc_info
Facebook: https://www.facebook.com/TheBusinessResearchCompany
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Blog: https://blog.tbrc.info/
Healthcare Blog: https://healthcareresearchreports.com/
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model