The Bleeding Disorder Testing Global Market Report 2024 by The Business Research Company provides market overview across 60+ geographies in the seven regions – Asia-Pacific, Western Europe, Eastern Europe, North America, South America, the Middle East, and Africa, encompassing 27 major global industries. The report presents a comprehensive analysis over a ten-year historic period (2010-2021) and extends its insights into a ten-year forecast period (2023-2033).
Learn More On The Bleeding Disorder Testing Market:
https://www.thebusinessresearchcompany.com/report/bleeding-disorder-testing-global-market-report
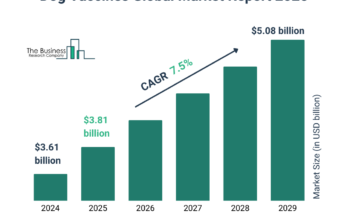
According to The Business Research Company’s Bleeding Disorder Testing Global Market Report 2024, The bleeding disorder testing market size has grown strongly in recent years. It will grow from $13.92 billion in 2023 to $14.99 billion in 2024 at a compound annual growth rate (CAGR) of 7.7%. The growth in the historic period can be attributed to the increasing prevalence of bleeding disorders, increasing investment in research and development, a growing number of specialized clinics, increasing demand for early diagnosis, and a rising number of clinical trials.
The bleeding disorder testing market size is expected to see strong growth in the next few years. It will grow to $20.40 billion in 2028 at a compound annual growth rate (CAGR) of 8.0%. The growth in the forecast period can be attributed to the increasing prevalence of hemophilia rising awareness of bleeding disorder symptoms, increasing investment in diagnostic research, rising healthcare spending globally, and rising prevalence of rare bleeding disorders. Major trends in the forecast period include technological advancements, integration of artificial intelligence, personalized medicine, telemedicine services, and home-based testing kits.
The increasing number of clinical trials is expected to propel the growth of the bleeding disorder testing market going forward. Clinical trials are research studies that assess the safety and efficacy of novel medical treatments, therapies, medicines, or technology in humans. The increase in clinical trials is due to advancements in medical research, growing investment in healthcare innovation, and rising demand for new treatments and therapies. Bleeding disorder testing is used in clinical trials to evaluate the efficacy and safety of new medicines and therapies for bleeding disorders, assess how well these treatments manage or correct clotting abnormalities, and monitor patient responses to ensure optimal dosing and effectiveness. For instance, according to ClinicalTrials.gov, a US-based clinical trial registry, around 477,228 clinical trials were registered at the end of 2023, an increase from 437,507 in 2022. Therefore, the increasing number of clinical trials is driving the growth of the bleeding disorder testing market.
Get A Free Sample Of The Report (Includes Graphs And Tables):
https://www.thebusinessresearchcompany.com/sample.aspx?id=18263&type=smp
The bleeding disorder testing market covered in this report is segmented –
1) By Product Type: Reagents And Consumables, Instruments
2) By Indication: Hemophilia A, Hemophilia B, Von Willebrand Disease, Idiopathic Thrombocytopenic Purpura, Other Indications
3) By Treatment Type: Factor Replacement Therapy, Drug Therapy
4) By End User: Hospitals And Clinics, Diagnostic Centers, Other End Users
Major companies operating in the bleeding disorder testing market are focused on developing innovative products, such as gene therapy for Hemophilia B, to provide long-term solutions, cure genetic bleeding disorders, enhance treatment outcomes, and expand their market reach. Hemophilia B is a type of bleeding disorder. It is characterized by a deficiency of clotting factor IX, which is essential for proper blood coagulation. Gene therapy for Hemophilia B is an emerging treatment aimed at providing a long-term solution by addressing the underlying genetic defect responsible for the condition. For instance, in February 2023, CSL Limited, an Australia-based biotechnology company, and the European Commission approved conditional marketing authorization (CMA) for HEMGENIX, the first and only one-time gene therapy for treating severe and moderately severe hemophilia B. This one-time treatment aims to provide long-lasting efficacy, reducing or eliminating the need for regular factor replacement therapy. HEMGENIX is notable for its innovative approach to gene therapy, offering a transformative option for patients with hemophilia B and potentially changing the standard of care in the field.
The bleeding disorder testing market report table of contents includes:
1. Executive Summary
2. Bleeding Disorder Testing Market Characteristics
3. Bleeding Disorder Testing Market Trends And Strategies
4. Bleeding Disorder Testing Market – Macro Economic Scenario
5. Global Bleeding Disorder Testing Market Size and Growth
.
.
.
32. Global Bleeding Disorder Testing Market Competitive Benchmarking
33. Global Bleeding Disorder Testing Market Competitive Dashboard
34. Key Mergers And Acquisitions In The Bleeding Disorder Testing Market
35. Bleeding Disorder Testing Market Future Outlook and Potential Analysis
36. Appendix
Contact Us:
The Business Research Company
Europe: +44 207 1930 708
Asia: +91 88972 63534
Americas: +1 315 623 0293
Email: [email protected]
Follow Us On:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
Twitter: https://twitter.com/tbrc_info
Facebook: https://www.facebook.com/TheBusinessResearchCompany
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Blog: https://blog.tbrc.info/
Healthcare Blog: https://healthcareresearchreports.com/
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model