The cell and gene therapy clinical trial services global market report 2024from The Business Research Company provides comprehensive market statistics, including global market size, regional shares, competitor market share, detailed segments, trends, and opportunities. This report offers an in-depth analysis of current and future industry scenarios, delivering a complete perspective for thriving in the industrial automation software market.
Cell And Gene Therapy Clinical Trial Services Market, 2024report by The Business Research Company offers comprehensive insights into the current state of the market and highlights future growth opportunities.
Market Size –
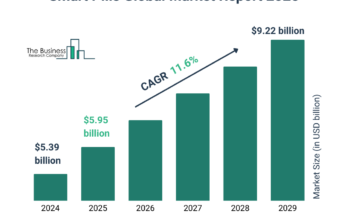
The cell and gene therapy clinical trial services market size has grown rapidly in recent years. It will grow from $3.44 billion in 2023 to $3.79 billion in 2024 at a compound annual growth rate (CAGR) of 10.2%. The growth in the historic period can be attributed to the increased prevalence of chronic diseases, increased demand for effective treatments for rare genetic disorders, increased government funding for research in regenerative medicine, increased understanding of molecular mechanisms underlying various diseases, and increased awareness of the potential of personalized medicine.
The cell and gene therapy clinical trial services market size is expected to see rapid growth in the next few years. It will grow to $5.64 billion in 2028 at a compound annual growth rate (CAGR) of 10.4%. The growth in the forecast period can be attributed to increasing global incidence of genetic and auto-immune diseases, rising public and private sector funding for gene therapy research, growing adoption of novel technologies, and a rise in the number of genetic defects. Major trends in the forecast period include the adoption of AI, the adoption of machine learning, the adoption of personalized medicine, technological advancements, and gene therapies.
Order your report now for swift delivery @
Scope Of Cell And Gene Therapy Clinical Trial Services MarketThe Business Research Company’s reports encompass a wide range of information, including:
1. Market Size (Historic and Forecast): Analysis of the market’s historical performance and projections for future growth.
2. Drivers: Examination of the key factors propelling market growth.
3. Trends: Identification of emerging trends and patterns shaping the market landscape.
4. Key Segments: Breakdown of the market into its primary segments and their respective performance.
5. Focus Regions and Geographies: Insight into the most critical regions and geographical areas influencing the market.
6. Macro Economic Factors: Assessment of broader economic elements impacting the market.
Cell And Gene Therapy Clinical Trial Services Market Overview
Market Drivers –
The rising incidence of genetic disease is expected to propel the growth of the cell and gene therapy clinical trial services market going forward. Genetic diseases are ailments caused by variations in a person’s DNA or genetic composition. The incidence of genetic diseases is on the rise due to numerous factors, including the increased availability of genetic testing, the occurrence of genetic mutations, and the prevalence of consanguineous marriage. Cell and gene therapy clinical trial services address the rising incidence of genetic diseases by encouraging the development of novel treatments, tailoring therapies to individual patients, accelerating the translation of research into clinical practice, and expanding the options available to patients suffering from genetic disorders. For instance, in July 2022, according to the Cystic Fibrosis (CF) Foundation, a US-based non-profit organization, there had been an upsurge in the number of people with cystic fibrosis. In 2022, cystic fibrosis affected almost 40,000 children and adults in the United States, and over 105,000 people worldwide have been diagnosed. Therefore, the rising incidence of genetic disease is driving the growth of the cell and gene therapy clinical trial services market.
Market Trends –
Major companies operating in the cell and gene therapy clinical trial services market are focusing their efforts on introducing advanced technology with customer relationship management system technology, to improve efficiency, and gain a competitive edge in the market. The customer relationship management system utilized by cell and gene therapy clinical trial services streamlines and coordinates interactions with trial participants, healthcare providers, and other stakeholders throughout the clinical trial process. For instance, in April 2023, AmerisourceBergen Corporation, a US-based healthcare company, launched the Cell and Gene Therapy (CGT) Integration Hub, a platform that aims to increase connectivity and streamline processes throughout the cell and gene therapy treatment pathway. This platform is backed by AmerisourceBergen’s customer relationship management (CRM) system and attempts to streamline the path-to-care process, in part by giving physicians and patient services teams better visibility across the therapy development and delivery process. The CGT Integration Hub, which includes features such as expedited benefits investigation, real-time status tracking, and proactive alerts, aims to simplify care coordination, minimize barriers, and improve the entire patient and provider experience when delivering cell and gene therapies.
The cell and gene therapy clinical trial services market covered in this report is segmented –
1) By Service: Clinical Trial Design And Planning, Supply And Logistic Services, Regulatory Affairs And Compliance, Data Management And Biostatics, Site Management And Monitoring, Other Services
2) By Therapy Type: Gene Therapy, Cell Therapy, Gene Modified Cell Therapy
3) By Indication: Oncology, Hematology, Metabolic Disorders, Infectious Diseases, Neurology, Cardiovascular Diseases, Musculoskeletal Disorders, Other Indications
4) By End-Use: Pharmaceutical And Biotechnology Companies, Contract Research Organizations, Academic And Research Institutes, Other End-Users
Get an inside scoop of the cell and gene therapy clinical trial services market, Request now for Sample Report @
Regional Insights –
North America was the largest region in the cell and gene therapy clinical trial services market in 2023. It is expected to be the fastest-growing region in the forecast period. The regions covered in the cell and gene therapy clinical trial services market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
Key Companies –
Major companies operating in the cell and gene therapy clinical trial services market are Thermo Fisher Scientific Inc., Sharp Services LLC, IQVIA, Laboratory Corporation of America Holdings, ICON plc, Syneos Health Inc., Catalent Pharma Solutions Inc., Charles River Laboratories International Inc., Parexel International Corporation, PRA Health Sciences Inc., Covance Inc., Medpace Holdings Inc., BioClinica Inc., Precision Medicine Group LLC, Worldwide Clinical Trials LLC, Clinigen Group plc, Evidera Inc., Advarra LLC, Veristat LLC, Clinipace Inc., Celonic AG, Cromsource Inc., Novotech Pty Ltd., MedSource Holdings Inc., Frontage Laboratories Inc.
Table of Contents
1. Executive Summary
2. Cell And Gene Therapy Clinical Trial Services Market Report Structure
3. Cell And Gene Therapy Clinical Trial Services Market Trends And Strategies
4. Cell And Gene Therapy Clinical Trial Services Market – Macro Economic Scenario
5. Cell And Gene Therapy Clinical Trial Services Market Size And Growth
…..
27. Cell And Gene Therapy Clinical Trial Services Market Competitor Landscape And Company Profiles
28. Key Mergers And Acquisitions
29. Future Outlook and Potential Analysis
30. Appendix
Contact Us:
The Business Research Company
Europe: +44 207 1930 708
Asia: +91 88972 63534
Americas: +1 315 623 0293
Email: info@tbrc.info
Follow Us On:
Twitter: https://twitter.com/tbrc_info
Blog: https://blog.tbrc.info/
Healthcare Blog: https://healthcareresearchreports.com/
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model