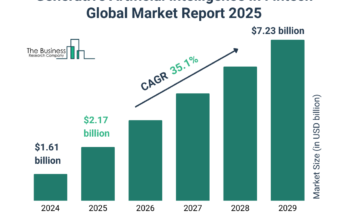
At what pace is the difficile-associated diarrhea treatment market growing, and what is its estimated value?
The difficile-associated diarrhea treatment market size has grown rapidly in recent years. It will grow from $3.27 billion in 2024 to $3.7 billion in 2025 at a compound annual growth rate (CAGR) of 13.2%. The growth in the historic period can be attributed to prevalence of hospital-acquired infections, aging population, increasing prevalence of clostridium difficile infections, increasing urbanization.
The difficile-associated diarrhea treatment market size is expected to see rapid growth in the next few years. It will grow to $5.8 billion in 2029 at a compound annual growth rate (CAGR) of 11.9%. The growth in the forecast period can be attributed to patient awareness, emerging therapies, global health initiatives, increasing demand for antimicrobial therapy, climate change, demand for self-administered treatments. Major trends in the forecast period include personalized treatment, telemedicine for follow-up, research into novel therapies, advancements in diagnostic techniques.
Get Your Free Sample of The Global Difficile-Associated Diarrhea Treatment Market Report:
https://www.thebusinessresearchcompany.com/sample.aspx?id=12930&type=smp
Which Market drivers have played a significant role in driving the difficile-associated diarrhea treatment market?
The increasing prevalence of digestive disorders is expected to propel the growth of the difficile-associated diarrhea treatment market going forward. Digestive disorders refer to a group of medical conditions that affect the normal functioning of the digestive system, which is responsible for breaking down food and absorbing nutrients. Clostridium difficile (C. diff) infection is associated with digestive disorders, specifically diarrhea and colitis. Clostridium difficile is a bacterium that can infect the bowel by producing toxins that cause inflammation and damage the intestinal lining. With more people being diagnosed with digestive disorders, there is an increasing need for effective treatments to manage associated symptoms like difficile-associated diarrhea. For instance, in June 2023, according to Crohn’s and Colitis Canada, a Canada-based non-profit organization, over 322,600 Canadians were estimated to live with inflammatory bowel diseases (IBD) in 2023, accounting for approximately 0.82% of the population. Further, it is estimated that around 470,000 Canadians will be living with IBD by 2035, about 1.1% of the population, or 1 in every 91 people in the country. Therefore, the increasing prevalence of digestive disorders is expected to propel the growth of the difficile-associated diarrhea treatment market.
What are the key segments within the difficile-associated diarrhea treatment market?
The difficile-associated diarrhea treatment market covered in this report is segmented –
1) By Type: Narrow Spectrum Antibiotics, Broad Spectrum Antibiotics
2) By Distribution Channel: Hospital Pharmacy, Online Pharmacy, Retail Pharmacy
3) By End User: Hospitals, Homecare, Specialty Clinics, Other End-Users
Subsegments:
1) By Narrow Spectrum Antibiotics: Vancomycin, Fidaxomicin, Metronidazole
2) By Broad Spectrum Antibiotics: Ampicillin, Ciprofloxacin, Clindamycin
Order your report now for swift delivery
https://www.thebusinessresearchcompany.com/report/difficile-associated-diarrhea-treatment-global-market-report
Which key players are shaping the difficile-associated diarrhea treatment market?
Major companies operating in the difficile-associated diarrhea treatment market are Pfizer Inc., F. Hoffmann-La Roche AG, Merck & Co. Inc., AbbVie Inc., Novartis AG, Sanofi S.A., Bristol-Myers Squibb Company, AstraZeneca PLC, Abbott Laboratories, GlaxoSmithKline PLC, Takeda Pharmaceutical Company Limited, Boehringer Ingelheim International GmbH, Teva Pharmaceutical Industries Ltd., Mylan N.V., Astellas Pharma Inc., Bausch Health Companies Inc., UCB S.A., Sun Pharmaceutical Industries Ltd., Perrigo Company PLC, Dr. Reddy’s Laboratories Ltd., Aurobindo Pharma Limited, Cipla Limited, Ferring B.V., Hikma Pharmaceuticals PLC, Amneal Pharmaceuticals LLC, Zydus Lifesciences Ltd., Lupin Limited, Torrent Pharmaceuticals Ltd., Salix Pharmaceuticals Inc., Almirall S.A.
Which transformative trends will shape the difficile-associated diarrhea treatment market landscape?
Major companies operating in the difficile-associated diarrhea treatment market are focused on developing innovative drugs and getting them approved to sustain their position in the market. Drug approvals for difficile-associated diarrhea treatment are increasing due to the need for more effective and safer treatments, such as SER-109, which provides a new therapeutic option for difficile-associated diarrhea treatment. For instance, in April 2023, the U.S. Food and Drug Administration, a US-based federal agency, approved SER-109, the first orally administered fecal microbiota product to prevent the recurrence of Clostridioides difficile diarrhea developed by Seres Therapeutics, a US-based biotech company. SER-109 is a live, spore-form probiotic administered orally in capsule form. It is composed of Firmicutes bacteria naturally found in the human gut. SER-109 works by restoring the balance of bacteria in the stomach, which can help prevent C. diff infections from recurring. This medication helps prevent the recurrence of Clostridioides difficile (C. diff) infection in individuals aged 18 and above following antibacterial treatment.
How do regional factors impact the difficile-associated diarrhea treatment market, and which region is the largest contributor?
North America was the largest region in the difficile-associated diarrhea treatment market in 2024. The regions covered in difficile-associated diarrhea treatment market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa.
What Does The Difficile-Associated Diarrhea Treatment Market Report 2025 Offer?
The difficile-associated diarrhea treatment market research report from The Business Research Company offers global market size, growth rate, regional shares, competitor analysis, detailed segments, trends, and opportunities.
Difficile-associated diarrhea is a bacterium that causes an infection of the large intestine (colon) and often infects people who have recently taken antibiotics. Difficile-associated diarrhea treatment is used to control the overgrowth of C. difficile bacteria (Clostridium) in the colon and reduce the production of toxins. Common symptoms include watery diarrhea, loss of appetite, nausea, abdominal pain and more.
Purchase the exclusive report now to unlock valuable market insights:
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=12930
About The Business Research Company
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead.
Our flagship product, the Global Market Model, is a premier market intelligence platform delivering comprehensive and updated forecasts to support informed decision-making.
Contact Us:
The Business Research Company
Europe: +44 207 1930 708
Asia: +91 88972 63534
Americas: +1 315 623 0293
Email: info@tbrc.info
Follow Us On:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
Twitter: https://twitter.com/tbrc_info
Facebook: https://www.facebook.com/TheBusinessResearchCompany
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Blog: https://blog.tbrc.info/
Healthcare Blog: https://healthcareresearchreports.com/
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model