How has the electronic data capture systems market evolved, and where is it heading next?
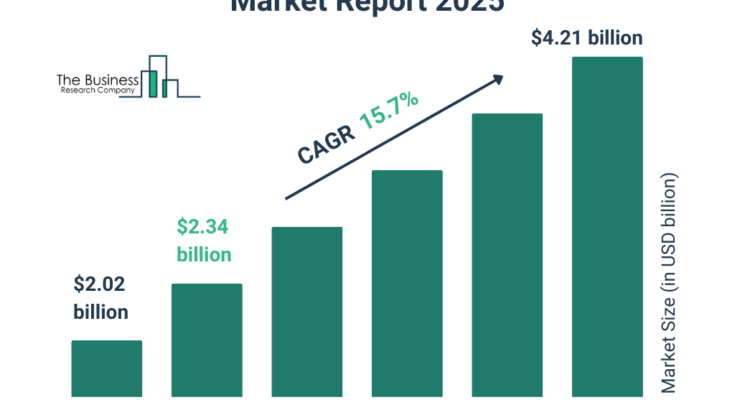
The electronic data capture systems market size has grown rapidly in recent years. It will grow from $2.02 billion in 2024 to $2.34 billion in 2025 at a compound annual growth rate (CAGR) of 16.1%. The growth in the historic period can be attributed to regulatory compliance, cost reduction initiatives, increasing clinical trial complexity, demand for real-time data access, globalization of clinical trials.
The electronic data capture systems market size is expected to see rapid growth in the next few years. It will grow to $4.21 billion in 2029 at a compound annual growth rate (CAGR) of 15.7%. The growth in the forecast period can be attributed to aging population and chronic diseases, personalized medicine and precision healthcare, adoption of risk-based monitoring, expansion of real-world evidence (RWE) studies, emerging markets growth. Major trends in the forecast period include increased emphasis on patient-centricity, integration of artificial intelligence and machine learning, expansion of decentralized clinical trials, growth of wearable technology integration, enhanced focus on data security and privacy.
Get Your Free Sample of The Global Electronic Data Capture Systems Market Report:
https://www.thebusinessresearchcompany.com/sample.aspx?id=15170&type=smp
How have varous drivers impacted the growth of the electronic data capture systems market?
The rising demand for outsourcing clinical trials to contract research organizations is expected to propel the growth of the electronic data capture systems market going forward. Clinical trial outsourcing refers to hiring external organizations or service providers to conduct various aspects of clinical trials on behalf of pharmaceutical, biotechnology, or medical device companies. The demand for outsourcing clinical trials is due to the cost efficiency, access to expertise, global reach, flexibility, scalability, and focus on core competencies that contract research organizations (CROs) provide. Electronic data capture systems are used in clinical trials to collect, manage, and analyze patient data digitally, streamlining the data collection process, improving accuracy, and facilitating real-time monitoring and reporting of trial outcomes. For instance, in October 2023, according to Applied Clinical Trials Online, a digital platform owned by MJH Life Sciences, a US-based medical media company, the utilization of functional service provider (FSP) outsourcing by large biopharma companies is experiencing annual growth of over 13%. Therefore, the rising demand for outsourcing clinical trials to clinical research organizations is driving the growth of the electronic data capture systems market.
What are the primary segments of the electronic data capture systems market?
The electronic data capture systems market covered in this report is segmented –
1) By Component: Software, Services
2) By Clinical Trial Phase: Phase I, Phase II, Phase III, Phase IV
3) By Delivery Mode: Cloud-Based (SAAS) Solutions, Web-Hosted (On-Demand) Solutions, Licensed Enterprise (On-Premise) Solutions
4) By End-User: Hospitals Or Healthcare providers, Contract Research Organizations, Pharmaceutical And Biotechnology Firms, Medical Device Firms, Other End-Users
Subsegments:
1) By Software: EDC Software Platforms, Cloud-Based EDC Software, Mobile EDC Software
2) By Services: Implementation Services, Consulting Services, Support And Maintenance Services, Training Services
Order your report now for swift delivery
Which firms are leading the electronic data capture systems market?
Major companies operating in the electronic data capture systems market report are International Business Machines Corporation (IBM); Oracle Corporation; IQVIA Inc.; Parexel International Corporation; Veeva Systems Inc.; Clario Medical Imaging; Medidata Solutions Inc.; Signant Health; Calyx International; ArisGlobal LLC; Kinapse Ltd.; Merge Healthcare Inc.; OmniComm Systems Inc.; MedNet Solutions; eClinical Solutions; BioForum Group; Acceliant Corporation; DATATRAK International Inc.; OpenClinica LLC; Castor EDC; DatStat Inc.; eResearchTechnology Inc.; Cmed Technology Ltd.; Clinerion Ltd.; Arithmos Srl.
How will industry trends affect the trajectory of the electronic data capture systems market?
Major companies operating in the electronic data capture systems market are focusing on developing innovative software solutions such as electronic data capture (EDC) platform for clinical trails. An electronic data capture (EDC) platform for clinical trials is a digital system used to collect and manage trial data efficiently, ensuring data accuracy, real-time access, and regulatory compliance. For instance, in March 2023, Cloudbyz, a US-based cloud technology company, introduced Cloudbyz EDC 2.0, an advanced Electronic Data Capture platform aimed at optimizing clinical research operations. The platform enhances operational efficiency, ensures data integrity, supports regulatory compliance, and accelerates the delivery of innovative treatments to patients. Cloudbyz EDC 2.0 simplifies data collection and management, reduces manual errors, and meets global regulatory standards, including FDA 21 CFR Part 11 and GDPR.
Which geographic trends are shaping the electronic data capture systems market, and which region has the highest market share?
North America was the largest region in the electronic data capture systems market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the electronic data capture systems market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
What Does The Electronic Data Capture Systems Market Report 2025 Offer?
The electronic data capture systems market research report from The Business Research Company offers global market size, growth rate, regional shares, competitor analysis, detailed segments, trends, and opportunities.
Electronic data capture systems (EDC) are computerized systems designed to collect, validate, and manage clinical trial data electronically. These systems streamline the data collection process, reduce errors, and improve data quality, leading to faster and more reliable clinical trial results. Electronic data capture systems (EDC) enable researchers to conduct studies more efficiently, ultimately contributing to developing new treatments and therapies.
Purchase the exclusive report now to unlock valuable market insights:
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=15170
About The Business Research Company
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead.
Our flagship product, the Global Market Model, is a premier market intelligence platform delivering comprehensive and updated forecasts to support informed decision-making.
Contact Us:
The Business Research Company
Europe: +44 207 1930 708
Asia: +91 88972 63534
Americas: +1 315 623 0293
Email: info@tbrc.info
Follow Us On:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
Twitter: https://twitter.com/tbrc_info
Facebook: https://www.facebook.com/TheBusinessResearchCompany
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Blog: https://blog.tbrc.info/
Healthcare Blog: https://healthcareresearchreports.com/
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model