The endotoxin testing global market report 2024 from The Business Research Company provides comprehensive market statistics, including global market size, regional shares, competitor market share, detailed segments, trends, and opportunities. This report offers an in-depth analysis of current and future industry scenarios, delivering a complete perspective for thriving in the industrial automation software market.
Endotoxin Testing Market, 2024 report by The Business Research Company offers comprehensive insights into the current state of the market and highlights future growth opportunities.
Market Size –
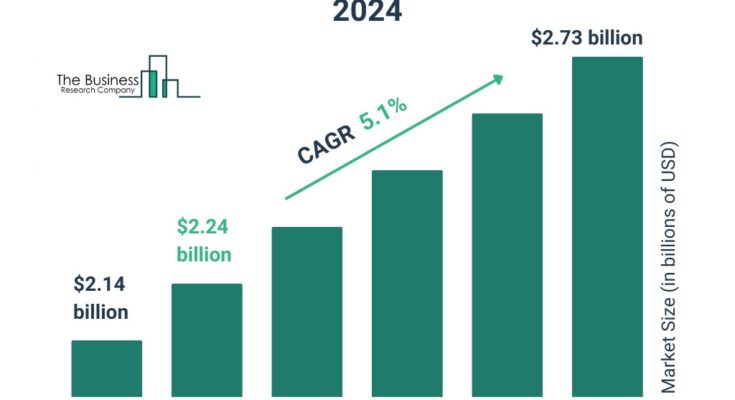
The endotoxin testing market size has grown steadily in recent years. It will grow from $2.14 billion in 2023 to $2.24 billion in 2024 at a compound annual growth rate (CAGR) of 4.6%. The growth in the historic period can be attributed to increasing demand for biologics and biosimilars, growing awareness about the importance of endotoxin testing, growing prevalence of infectious diseases.
The endotoxin testing market size is expected to see strong growth in the next few years. It will grow to $2.73 billion in 2028 at a compound annual growth rate (CAGR) of 5.1%. The growth in the forecast period can be attributed to expansion of the biopharmaceutical industry, increasing investment in research and development of endotoxin testing, global healthcare expansion. Major trends in the forecast period include innovative endotoxin detection tools, adoption of endotoxin testing service, demand for endotoxin testing services.
Order your report now for swift delivery @
https://www.thebusinessresearchcompany.com/report/endotoxin-testing-global-market-report
Scope Of Endotoxin Testing Market
The Business Research Company’s reports encompass a wide range of information, including:
1. Market Size (Historic and Forecast): Analysis of the market’s historical performance and projections for future growth.
2. Drivers: Examination of the key factors propelling market growth.
3. Trends: Identification of emerging trends and patterns shaping the market landscape.
4. Key Segments: Breakdown of the market into its primary segments and their respective performance.
5. Focus Regions and Geographies: Insight into the most critical regions and geographical areas influencing the market.
6. Macro Economic Factors: Assessment of broader economic elements impacting the market.
Endotoxin Testing Market Overview
Market Drivers –
The increasing number of healthcare-associated infections is expected to propel the growth of the endotoxin testing market going forward. Healthcare-associated infections refer to infections acquired while receiving medical care that were not present at the time of admission to the hospital. Healthcare-associated infections often arise from contaminated medical equipment or procedures, necessitating rigorous testing to ensure patient safety. Endotoxin testing services significantly prevent healthcare-associated infections by assessing the bacterial endotoxin levels in medical equipment, especially injectable pharmaceutical products and implantable medical devices. For instance, in December 2022, according to the United Nations International Children’s Emergency Fund (UNICEF), a US-based agency of the United Nations, pneumonia was the infectious disease that killed the most children, taking the lives of over 700,000 of them annually, or around 2,000 per day. Furthermore, in May 2022, according to the World Health Organization, a Switzerland-based agency of the United Nations, 24% of patients are affected by healthcare-associated sepsis every year and 52.3% of those patients treated in an intensive care unit die each year. Therefore, the increasing number of healthcare-associated infections is driving the growth of the endotoxin testing market.
Market Trends –
Major companies operating in the endotoxin testing market are focused on adopting new technological solutions to sustain their position in the market. For instance, in August 2023, Lonza Group AG, a Switzerland-based pharmaceutical, biotechnology and nutrition manufacturing company, launched the Nebula absorbance reader, a new absorbance microplate reader for streamlined endotoxin and pyrogen testing. The instrument is fully connected with Lonza’s most recent WinKQCL software to facilitate data integrity compliance, speed training and lessen validation load. There is no longer a requirement for WinKQCL software users to learn new software to utilize the contemporary reader, reducing training demands. The Nebula Absorbance Reader is an innovative and technologically advanced replacement reader that produces outcomes similar to those frequently observed with the ELx808, the industry-standard absorbance reader that Lorenz no longer offers for sale. Additionally, it is designed to be compatible with and adhere to the exacting requirements of Lonza’s absorbance-based endotoxin assays, including the Lonza PYROGENT 5000 Turbidimetric and Kinetic-QCL Chromogenic Endotoxin Assays.
The endotoxin testing market covered in this report is segmented –
1) By Test Type: LAL (Limulus Amebocyte Lysate) Test; Chromogenic Tests; Turbidimetric Tests; Gel Clot Tests; MAT Test; Rabbit Pyrogen Test; Recombinant Factor C (rFC) Assay
2) By Application: Medical Devices; Pharmaceuticals; Packaging; Raw Materials
3) By End-User: Hospitals; Laboratories; Research Institutes
Get an inside scoop of the endotoxin testing market, Request now for Sample Report @
https://www.thebusinessresearchcompany.com/sample.aspx?id=12932&type=smp
Regional Insights –
North America was the largest region in the endotoxin testing market in 2023. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in endotoxin testing market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa.
Key Companies –
Major players in the endotoxin testing market are Thermo Fisher Scientific Inc., Merck KGaA, bioMérieux SA., Eurofins Scientific SE, Lonza Group Ltd., WuXi AppTec Co. Ltd., Bio-Rad Laboratories Inc., Maravai LifeSciences Holdings Inc., Cambrex Corporation, GenScript Biotech Corporation, Charles River Laboratories International Inc., Nelson Laboratories LLC, Lifecore Biomedical Inc., Stellar Biotechnologies Inc., Biovision Inc., Associates of Cape Cod Inc., FUJIFILM Wako Pure Chemical Corporation, InvivoGen Inc., Tebu-bio Nv., MatTek Corporation, Microcoat Biotechnologie GmbH, Accelagen Inc., BioAssay Systems LLC, BioThema AB, Hycult Biotech Inc., Pacific Biolabs Inc., Xiamen Bioendo Technology Co. Ltd., Zhanjiang A&C Biological Ltd., Accugen Laboratories Inc.
Table of Contents
1. Executive Summary
2. Endotoxin Testing Market Report Structure
3. Endotoxin Testing Market Trends And Strategies
4. Endotoxin Testing Market – Macro Economic Scenario
5. Endotoxin Testing Market Size And Growth
…..
27. Endotoxin Testing Market Competitor Landscape And Company Profiles
28. Key Mergers And Acquisitions
29. Future Outlook and Potential Analysis
30. Appendix
Contact Us:
The Business Research Company
Europe: +44 207 1930 708
Asia: +91 88972 63534
Americas: +1 315 623 0293
Email: [email protected]
Follow Us On:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
Twitter: https://twitter.com/tbrc_info
Facebook: https://www.facebook.com/TheBusinessResearchCompany
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Blog: https://blog.tbrc.info/
Healthcare Blog: https://healthcareresearchreports.com/
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model