The hereditary breast and ovarian cancer syndrome treatment global market report 2024 from The Business Research Company provides comprehensive market statistics, including global market size, regional shares, competitor market share, detailed segments, trends, and opportunities. This report offers an in-depth analysis of current and future industry scenarios, delivering a complete perspective for thriving in the industrial automation software market.
Hereditary Breast and Ovarian Cancer Syndrome Treatment Market, 2024 report by The Business Research Company offers comprehensive insights into the current state of the market and highlights future growth opportunities.
Market Size –
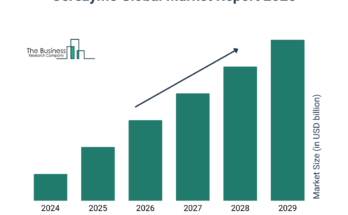
The hereditary breast and ovarian cancer syndrome treatment market size has grown strongly in recent years. It will grow from $6.28 billion in 2023 to $6.72 billion in 2024 at a compound annual growth rate (CAGR) of 7.1%. The growth in the historic period can be attributed to increased awareness and screening, supportive government policies, patient advocacy and empowerment, collaboration in research.
The hereditary breast and ovarian cancer syndrome treatment market size is expected to see strong growth in the next few years. It will grow to $8.57 billion in 2028 at a compound annual growth rate (CAGR) of 6.3%. The growth in the forecast period can be attributed to growing role of artificial intelligence, expanded access to genetic counseling, regulatory approvals for targeted therapie, personalized treatment plans, international collaborations in research. Major trends in the forecast period include gene editing technologies, advancements in immunotherapy, technological advances in oncology, immunotherapy for brca-related cancers, comprehensive multidisciplinary care.
Order your report now for swift delivery @
Scope Of Hereditary Breast and Ovarian Cancer Syndrome Treatment Market
The Business Research Company’s reports encompass a wide range of information, including:
1. Market Size (Historic and Forecast): Analysis of the market’s historical performance and projections for future growth.
2. Drivers: Examination of the key factors propelling market growth.
3. Trends: Identification of emerging trends and patterns shaping the market landscape.
4. Key Segments: Breakdown of the market into its primary segments and their respective performance.
5. Focus Regions and Geographies: Insight into the most critical regions and geographical areas influencing the market.
6. Macro Economic Factors: Assessment of broader economic elements impacting the market.
Hereditary Breast and Ovarian Cancer Syndrome Treatment Market Overview
Market Drivers –
The rise in genetic disorders is expected to propel the growth of the hereditary breast and ovarian cancer syndrome treatment market going forward. Newborn screening is a physical examination that screens all babies shortly after birth for specific health conditions, which health professionals use to identify and treat specific requirements before they make a baby sick. Newborn screening could identify babies at high risk of developing breast cancer and ovarian cancer, allowing them to start preventive measures early. For instance, in 2022, according to the Texas Department of State Health Services, a US-based state health agency, the total specimens received for newborn screening increased by 4.3% from 729,347 in 2021 to 761,000 in 2022. Further, around 394,000 babies were screened, and 1,130 babies were affected by one of the NBS disorders in 2022. Therefore, the rise in genetic disorders is driving the growth of the hereditary breast and ovarian cancer syndrome treatment market.
Market Trends –
Major companies operating in the hereditary breast and ovarian cancer syndrome treatment market are adopting new treatment options, such as comprehensive risk tests, to provide reliable services to the customers. A complete risk assessment test is a tool that helps to identify an individual’s risk of developing a disorder or experiencing a symptom of a disease. For instance, in February 2023, Genetic Technologies Limited, an Australia-based molecular diagnostics company, launched a ground-breaking comprehensive risk assessment test, a type of treatment for breast and ovarian cancer. It combines gene mutation testing for breast and ovarian cancers, hereditary and non-hereditary risk factors, and the GeneType risk score for both cancers. It has a unique approach that combines the detection of the 13 primary actionable breast and ovarian cancer susceptibility genes with the GeneType test platform. The new test platform addresses women over 30 who, outside of unusual genetic alterations, are at higher risk of developing breast and ovarian cancer in the general population.
The hereditary breast and ovarian cancer syndrome treatment market covered in this report is segmented –
1) By Treatment: Hormonal Therapy, Chemoprevention, Genetic Counselling, Medication, Other Treatments
2) By Diagnosis: Genetic Testing, Magnetic Resonance Imaging (MRI), Mammography, Other Diagnosis
3) By Route Of Administration: Oral, Parenteral, Other Routes Of Administration
4) By End-User: Hospitals, Specialty Clinics, Homecare, Other End-Users
Get an inside scoop of the hereditary breast and ovarian cancer syndrome treatment market, Request now for Sample Report @
Regional Insights –
North America was the largest region in the hereditary breast and ovarian cancer syndrome treatment market in 2023. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the hereditary breast and ovarian cancer syndrome treatment market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa
Key Companies –
Major companies operating in the hereditary breast and ovarian cancer syndrome treatment market report are Pfizer Inc., Johnson & Johnson Private Limited, F. Hoffmann-La Roche Ltd., Merck & Co., AbbVie Inc., Bayer AG, Sanofi-Aventis LLC , AstraZeneca plc, Abbott Laboratories, GlaxoSmithKline plc, Eli Lilly and Company, Teva Pharmaceutical Industries Ltd., Daiichi Sankyo Co. Ltd., Vertex Pharmaceuticals Inc., Mercy Health, Hikma Pharmaceuticals plc, Amneal Pharmaceuticals LLC, Lupin Limited , Seagen Inc., LEO Pharma Inc., LifeLabs Genetics, Natera Inc., Fresenius Kabi AG, Myriad Genetics Inc., Invitae Corporation, Editas Medicine Inc., GenMark Diagnostics Inc., Genentech Inc., CRISPR Therapeutics AG, Advocate Health Care
Table of Contents
1. Executive Summary
2. Hereditary Breast and Ovarian Cancer Syndrome Treatment Market Report Structure
3. Hereditary Breast and Ovarian Cancer Syndrome Treatment Market Trends And Strategies
4. Hereditary Breast and Ovarian Cancer Syndrome Treatment Market – Macro Economic Scenario
5. Hereditary Breast and Ovarian Cancer Syndrome Treatment Market Size And Growth
…..
27. Hereditary Breast and Ovarian Cancer Syndrome Treatment Market Competitor Landscape And Company Profiles
28. Key Mergers And Acquisitions
29. Future Outlook and Potential Analysis
30. Appendix
Contact Us:
The Business Research Company
Europe: +44 207 1930 708
Asia: +91 88972 63534
Americas: +1 315 623 0293
Email: [email protected]
Follow Us On:
Twitter: https://twitter.com/tbrc_info
Blog: https://blog.tbrc.info/
Healthcare Blog: https://healthcareresearchreports.com/
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model