How much is the intravenous fluid transfer drug devices market worth, and how is it expected to expand?
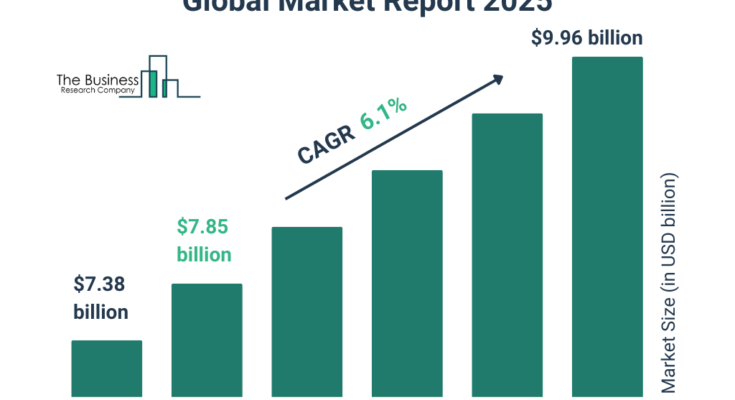
The intravenous fluid transfer drug devices market size has grown strongly in recent years. It will grow from $7.38 $ billion in 2024 to $7.85 $ billion in 2025 at a compound annual growth rate (CAGR) of 6.4%. The growth in the historic period can be attributed to increase in demand for efficient medical supplies, growth of healthcare infrastructure, rise in surgical interventions requiring IV support, policy changes, increase in healthcare infrastructure, and increase in new drugs requiring precise administration.
The intravenous fluid transfer drug devices market size is expected to see strong growth in the next few years. It will grow to $9.96 $ billion in 2029 at a compound annual growth rate (CAGR) of 6.1%. The growth in the forecast period can be attributed to growing demand for portable devices, increasing spending on advanced medical devices, rising demand for affordable healthcare solutions, and expanding non-hospital treatment settings. Major trends in the forecast period include integration of smart technologies, adoption of automated IV fluid transfer systems, advancements in safety features to reduce medication errors, and adoption of devices compatible with remote monitoring solutions.
Get Your Free Sample of The Global Intravenous Fluid Transfer Drug Devices Market Report:
https://www.thebusinessresearchcompany.com/sample.aspx?id=19535&type=smp
Which industry factors have accelerated the intravenous fluid transfer drug devices market’s expansion?
The rising hospitalization rate is expected to propel the growth of the intravenous fluid transfer drug device market going forward. The rising hospitalization rate is driven by an aging population, chronic illnesses, advanced diagnostics, and increased healthcare access, alongside lifestyle-related diseases and medical technology advancements. Fluid transfer systems ensure precise and controlled administration of fluids and medications directly into the bloodstream in hospitalized patients. For instance, in May 2023, according to a report published by the American Hospital Association (AHA), a US-based healthcare trade organization representing hospitals and healthcare networks, the total number of admissions in all U.S. hospitals reached 34,011,386 compared to 33,356,853 in 2022. Therefore, a rising hospitalization rate will drive intravenous fluid transfer drug device market growth.
What are the primary segments of the intravenous fluid transfer drug devices market?
The intravenous fluid transfer drug devicesmarket covered in this report is segmented –
1) By Type: Infusion Bags, Infusion Devices, Other Types
2) By Application: Autoimmune Diseases, Blood Disorders, Cardiovascular Disorders, Neurology, Oncology, Other Applications
3) By End-Use Sector: Hospitals, Specialized Clinics, Other End-Use Sectors
Subsegments:
1) By Infusion Bags: PVC Infusion Bags, Non-PVC Infusion Bags, Pre-Filled Infusion Bags, Multi-Chamber Infusion Bags, IV Fluid Administration Bags
2) By Infusion Devices: Infusion Pumps, Syringe Pumps, Ambulatory Infusion Pumps, Gravity Infusion Devices, Smart Infusion Devices
3) By Other Types: IV Sets And Accessories, IV Catheters, Needleless Connectors, Flow Regulators, Pressure Infusion Devices.
Order your report now for swift delivery
Which firms are leading the intravenous fluid transfer drug devices market?
Major companies operating in the intravenous fluid transfer drug devices market are Cardinal Health Inc., 3M Company, Medtronic plc, Becton, Dickinson and Company, Baxter International Inc., B. Braun Holding GmbH & Co. KG, Fresenius Kabi AG, Mindray Medical International Limited, Nipro Corporation, Moog Inc., West Pharmaceutical Services Inc., ICU Medical Inc., Insulet Corporation, Merit Medical Systems Inc., Avanos Medical Inc., Vygon SAS, NxStage Medical Inc., AngioDynamics Inc., Elcam Medical Inc., InfuSystem Holdings Inc., CODAN US Corporation, Zyno Medical LLC, Sippex, Arcomed AG
Which market trends are set to define the future of the intravenous fluid transfer drug devices market?
Major companies in the intravenous fluid transfer drug device market are developing modular and comprehensive infusion systems with features such as auto-identification to enhance accuracy, improve patient safety, and streamline the administration of fluids and medications. Auto-identification in infusion systems automatically verifies and matches medications to the correct patient using barcode scanning or RFID technology. For instance, in July 2023, Becton, Dickinson, and Company, a US-based medical device company, received FDA 510(k) clearance for its updated BD Alaris Infusion System. The BD Alaris Infusion System has updated hardware for its Point-of-Care Unit (PCU), including large volume pumps, syringe pumps, patient-controlled analgesia (PCA) pumps, respiratory monitoring, and auto-identification modules. It also introduces an advanced software version with improved cybersecurity and interoperability features that allow for seamless integration with widely used electronic medical record (EMR) systems, supporting smart and connected healthcare.
Which geographic trends are shaping the intravenous fluid transfer drug devices market, and which region has the highest market share?
North America was the largest region in the intravenous fluid transfer drug devices market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the intravenous fluid transfer drug devices market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
What Does The Intravenous Fluid Transfer Drug Devices Market Report 2025 Offer?
The intravenous fluid transfer drug devices market research report from The Business Research Company offers global market size, growth rate, regional shares, competitor analysis, detailed segments, trends, and opportunities.
Intravenous fluid transfer drug devices are tools used to deliver fluids and medications directly into a patient’s bloodstream via an intravenous line. The device ensures efficient, controlled, and immediate administration of treatments, which is crucial for hydration, medication delivery, and critical care. These devices are designed to prevent contamination of both the medication and the IV fluid, maintaining sterility and reducing the risk of infection.
Purchase the exclusive report now to unlock valuable market insights:
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=19535
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead.
Our flagship product, the Global Market Model, is a premier market intelligence platform delivering comprehensive and updated forecasts to support informed decision-making.
Contact Us:
The Business Research Company
Europe: +44 207 1930 708
Asia: +91 88972 63534
Americas: +1 315 623 0293
Email: info@tbrc.info
Follow Us On:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
Twitter: https://twitter.com/tbrc_info
Facebook: https://www.facebook.com/TheBusinessResearchCompany
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Blog: https://blog.tbrc.info/
Healthcare Blog: https://healthcareresearchreports.com/
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model