What is the current market size and future outlook for the pharmacovigilance and drug safety software market?
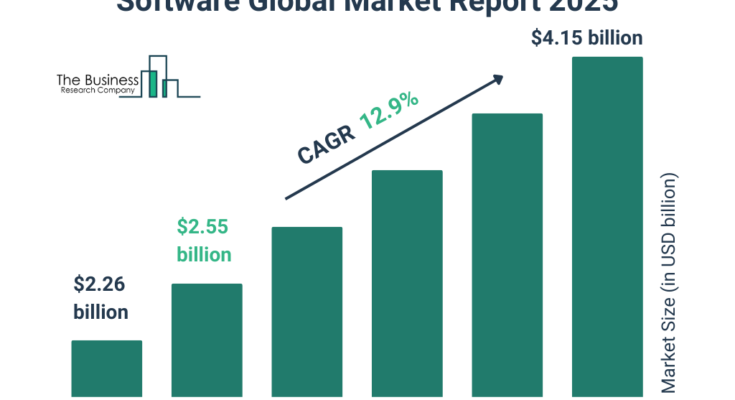
The pharmacovigilance and drug safety software market size has grown rapidly in recent years. It will grow from $2.26 billion in 2024 to $2.55 billion in 2025 at a compound annual growth rate (CAGR) of 13.2%. The growth in the historic period can be attributed to increasing the demand for pharmacovigilance and drug safety software.
The pharmacovigilance and drug safety software market size is expected to see rapid growth in the next few years. It will grow to $4.15 billion in 2029 at a compound annual growth rate (CAGR) of 12.9%. The growth in the forecast period can be attributed to rising adverse drug reaction incidences and the globalization of pharmacovigilance, growing incidence of adverse drug reactions, increased complexity in drug safety regulations, increase in the development of new drugs and therapies, increasing incidence of adverse drug reactions (ADRs), rising number of adverse drug reactions. Major trends in the forecast period include automation and AI integration, cloud-based solutions, technological innovation, advancement in technologies, and increasing healthcare digitalization.
Get Your Free Sample of The Global Pharmacovigilance And Drug Safety Software Market Report:
https://www.thebusinessresearchcompany.com/sample.aspx?id=19140&type=smp
How has the pharmacovigilance and drug safety software market evolved, and what factors have shaped its growth?
The growing demand for personalized medicine is expected to propel the growth of the pharmacovigilance and drug safety software market going forward. Personalized medicine, also known as precision medicine, is a medical approach that tailors healthcare decisions and treatments to the individual characteristics of each patient. The growing demand for personalized medicine is due to several factors, such as improved treatment outcomes, a focus on prevention and early detection, and cost-effectiveness. Pharmacovigilance and drug safety software are essential to the success of personalized medicine. By incorporating pharmacogenetics, real-time data analysis, post-marketing surveillance, and personalized medication information, these tools improve the safety and efficacy of individualized treatments while supporting robust risk management strategies and addressing ethical considerations. For instance, in February 2024, according to the Personalized Medicine Coalition, a US-based non-profit organization, the FDA approved 16 novel personalized therapies for patients with rare diseases in 2023, compared to 6 in 2022. Therefore, the growing demand for personalized medicine is driving the growth of the pharmacovigilance and drug safety software market.
What are the major segments of the pharmacovigilance and drug safety software market?
The pharmacovigilance and drug safety softwaremarket covered in this report is segmented –
1) By Software Type: Adverse Event Reporting Software, Drug Safety Audits Software, Issue Tracking Software, Fully Integrated Software
2) By Delivery Mode: On-premise, Cloud-based
3) By End User: Pharmaceutical And Biotechnology Companies, Contract Research Organizations, Business Process Outsourcing Firms, Other End Users
Subsegments:
1) By Adverse Event Reporting Software: Spontaneous Reporting Systems, EHR Integration Solutions, Mobile Reporting Applications
2) By Drug Safety Audits Software: Audit Management Solutions, Compliance Tracking Tools, Reporting And Analytics Modules
3) By Issue Tracking Software: Incident Reporting Systems, Workflow Management Tools, Collaboration Platforms
4) By Fully Integrated Software: Pharmacovigilance Platforms, Regulatory Compliance Systems, Data Analytics And Visualization Tools
Order your report now for swift delivery
Which companies dominate the pharmacovigilance and drug safety software market?
Major companies operating in the pharmacovigilance and drug safety software market are Accenture plc, International Business Machines Corporation, Deloitte Touche Tohmatsu Limited, Oracle Corporation, Capgemini SE, Cognizant Technology Solutions, IQVIA, Wipro Limited, Genepact, Parexel Academy, PharmaLex, Indegene Limited, RXLogix, Sarjen Systems Pvt Ltd., Anju Software Inc., Ennov Group, EXTEDO GmbH, ArisGlobal, AB Cube, Clinevo Technologies
How will evolving trends contribute to the growth of the pharmacovigilance and drug safety software market?
Major companies operating in the pharmacovigilance and drug safety software market are developing innovative technologies, such as cloud-based data lake platforms, to enhance patient safety, monitor adverse events more effectively, and improve regulatory compliance in clinical trials and post-market surveillance of medical devices. A cloud-based data lake platform is a scalable, centralized storage system that allows organizations to store, manage, and analyze vast amounts of structured and unstructured data. It provides real-time data access and supports advanced analytics and AI/ML workloads. For instance, in December 2023, Thermo Fisher Scientific Inc., a US-based biotechnology company, launched CorEvidence, an innovative proprietary cloud-based data lake platform designed to enhance pharmacovigilance processes within clinical research registries. This platform aims to optimize case processing and safety data management, particularly for post-authorization safety studies.
What are the key regional dynamics of the pharmacovigilance and drug safety software market, and which region leads in market share?
North America was the largest region in the pharmacovigilance and drug safety software market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the pharmacovigilance and drug safety software market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
What Does The Pharmacovigilance And Drug Safety Software Market Report 2025 Offer?
The pharmacovigilance and drug safety software market research report from The Business Research Company offers global market size, growth rate, regional shares, competitor analysis, detailed segments, trends, and opportunities.
Pharmacovigilance and drug safety software refers to a suite of digital tools designed to monitor, analyze, and report adverse drug reactions (ADRs) and other safety-related information associated with pharmaceuticals and medical products. This software plays a critical role in ensuring patient safety and regulatory compliance throughout the lifecycle of a drug, from development to post-marketing surveillance.
Purchase the exclusive report now to unlock valuable market insights:
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=19140
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead.
Our flagship product, the Global Market Model, is a premier market intelligence platform delivering comprehensive and updated forecasts to support informed decision-making.
Contact Us:
The Business Research Company
Europe: +44 207 1930 708
Asia: +91 88972 63534
Americas: +1 315 623 0293
Email: info@tbrc.info
Follow Us On:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
Twitter: https://twitter.com/tbrc_info
Facebook: https://www.facebook.com/TheBusinessResearchCompany
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Blog: https://blog.tbrc.info/
Healthcare Blog: https://healthcareresearchreports.com/
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model